H2O oxygen is the central atom and can either form one double or. Each oxygen atom needs to have 8 surrounding it so it can satisfy the octet rule.
How To Determine The Lewis Dot Structure Of O2 Quora
It can be designated as a homonuclear covalent double bond.

Is o2 double bonded. Oxygen has an electronic configuration of 8 that is its atomic number is 1s2 2s2 2p4. Find how many electrons are needed. Both the oxygen and the carbon now have an octet of electrons so this is an acceptable Lewis electron structure.
Jun 16 2021 In contrast silicon in group 14 has little tendency to form discrete siliconoxygen double bonds. Some simple molecules containing double bonds. As a result it needs to share two pair with the other oxygen atom.
Consequently SiO 2 has a three-dimensional network structure in which each silicon atom forms four SiO single bonds which makes the physical and chemical properties of SiO 2 very different from those of CO 2. It is two for each oxygen atom. Now in water ie.
When it forms a single bond it has six lone electrons and two shared electrons. The O 2 molecule is diatomic meaning that two atoms of the same element are connected in a pair. Oxygen atoms can form double bonds and nitrogen atoms can form triple bonds to ma.
It is four for one O2 molecule. Subjects Arts and Humanities. Find total valence electrons.
Hydroxyl group bonded to a tetrahedral carbon atom F. Jul 06 2021 Two atoms form double bonds to complete their octet and form a linear geometrical structure. Properties of molecularoxygen O2 suggest this moleculeis a diradical with an oxygen-oxygen bond order 1 ie a single bond and one unpaired electron on each oxygen atom.
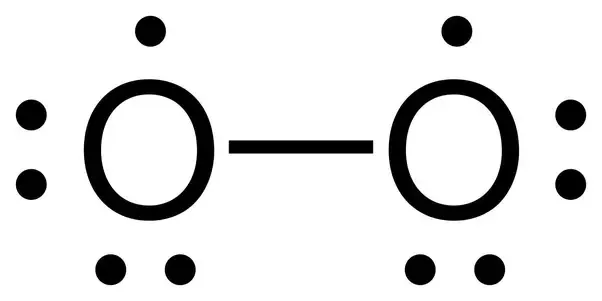
Below is the image of the geometrical structure of the Oxygen gas molecule Nature of Bond in O2. The bond angle formed between both Oxygen atoms is 180 degrees. Alkynes contain a carbon-carbon triple bond.
Contain a carbon-carbon double bond C. A double covalent bond is where two pairs of electrons are shared between the atoms rather than just one pair. When two oxygen atoms come together to form a bond they end up forming a double bond that is covalent in nature ie.
In a normal molecule carbon and oxygen form a double bond. Indeed in the molecular orbitals of O2 there are 8 electrons in two sigma and two pi bonding orbitals and 4 electrons in one sigma and two pi antibonding orbitals each of the pi antibonding orbitals hosting an unpaired electron. This video shows chemical bonds inside human body respiration.
Two oxygen atoms can both achieve stable structures by sharing two pairs of electrons as in the diagram. There is equal sharing of valence electrons. That is carbon contributes its 2 electrons and oxygen contributes its two electrons to form a double bond.
The O 2 Lewis structure has a double bond between two oxygen atoms. Ethers contain an oxygen atom bonded to two carbon atom groups by single bonds. The moleculedoes not have an oxygen-oxygen double.
Amines contain an amino group a N atom bonded to 1 2 or 3 carbon atom. Its called dioxygen O X 2 and its MO scheme is exactly the same as above except that. O2 Lewis Structure Setup.
In order to look at the double bond we want to find a species that has an O O bond order of 2. Oxygen has six valence electrons. To avoid having a double bond it would need 14.
Nov 28 2016 The diatomic oxygen molecule contains a total of 12 valence electrons spread amongst two atoms. Easy to use and portable study sets in Double Bonded Oxygen are great for studying in the way that works for you at the time that works for you. Jun 30 2021 O2 being a diatomic molecule forms a linear geometrical structure held by a double bond.
Oct 21 2015 The formal charge for oxygen is zero when it has a double bond because the formal charge equals the valence minus the sum of the lone electrons and the quotient of the shared electrons and 2 FCVE- L S2. Thats not hard at all. Feb 28 2016 How about the double bonds.
Mar 12 2020 Similar is the case with C It needs 4 more electrons for which it has a tendency to form either 4 single bonds 2 double bonds or one single and one triple bond. To give carbon an octet of electrons we use one of the lone pairs of electrons on oxygen to form a carbonoxygen double bond. The O has two bonding pairs and two lone pairs and C has four bonding pairs.
According to the octet rule oxygen atoms need to bond twice. Steps of drawing Lewis diagram. The double bond is shown conventionally by two lines joining the atoms.

Covalent Compounds Manoa Hawaii Edu Exploringourfluidearth

Why Aren T The Carbon Carbon Four Bonds Or C2 Molecules Found Quora